OB healthcare providers are true heroes and we’re here to support them with technology that enhances confidence, precision, and patient care
We are dedicated to advancing maternal health through innovative, minimally invasive solutions. Our device is designed to provide real-time insights into labor progress, helping clinicians make informed decisions that support mothers and babies throughout childbirth.
Streamline Your Workflow and Efficiency
Traditional labor assessment tools are essential but were not originally designed to generate structured, trendable measurements across care transitions, particularly in low-resource or high-workload settings where continuity and documentation can be constrained.
Remote Patient Assessment
Nurse-Operated Efficiency: The device is designed for use by trained healthcare professionals, including nurses, enabling standardized assessments without requiring continuous physician supervision. This supports efficient workflow and consistent patient monitoring.
Remote Monitoring Options: Where clinically validated and permitted, the device may allow remote assessment or data review by healthcare providers. Such functionality can support individualized labor management and improved coordination of care.
This information is intended for educational purposes only and does not constitute medical advice or promotion. Any remote or home-use features are subject to regulatory review.
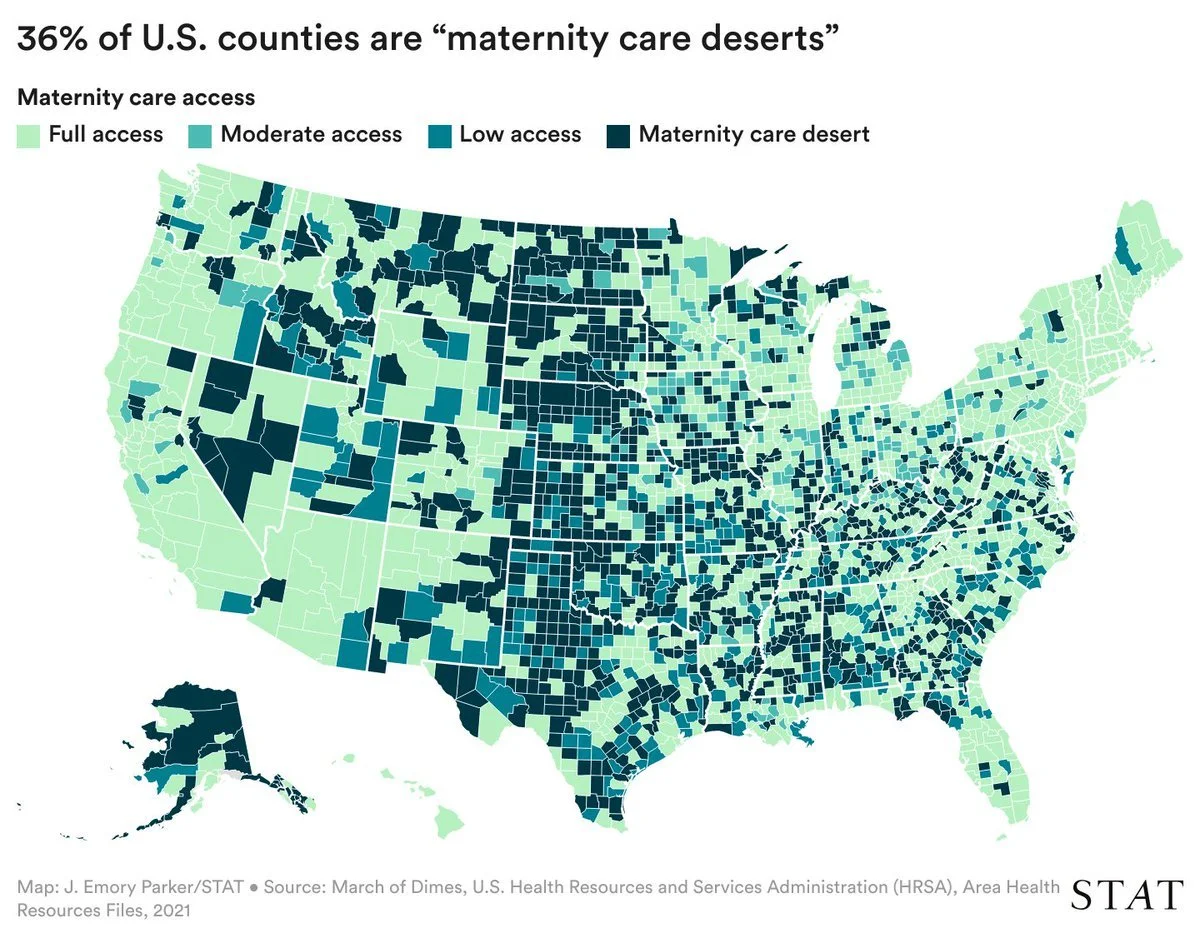
Maternal Care Deserts
According to March of Dimes, more than 35% of U.S. counties are classified as maternal care deserts or low-access areas, affecting over 5 million women of reproductive age. In these settings, obstetric care is often delivered with limited staffing, reduced specialist availability, and frequent handoffs, while labor assessment and documentation must still support timely clinical decisions.
Lidavex research focuses on understanding whether structured, objective approaches to cervical assessment could help support documentation, continuity, and team communication in these constrained environments—without altering clinical judgment or standards of care.
CerviLite is investigational and has not yet been cleared or approved by the U.S. Food and Drug Administration (FDA). Information provided is for educational purposes only and does not constitute medical advice or product promotion.